The majority of postpartum hemorrhage cases are caused by uterine atony.
The JADA System is intended to provide control and treatment of abnormal postpartum uterine bleeding or hemorrhage when conservative management is warranted.



JADA is made of silicone. A survey found that it is easy to use (98%, n=105/107).1

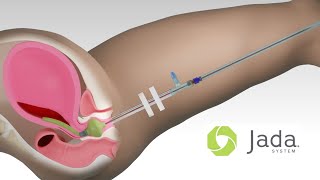
An Intrauterine Loop has interior Vacuum Pores inside a protective Shield to evacuate blood.

An expandable Cervical Seal is filled with sterile fluid to create a seal that holds a vacuum within the uterus.

A connector at the end of the tube is attached to a regulated vacuum source.

Before using JADA, please read the contraindications, warnings, precautions, and other important information in the accompanying Instructions for Use.
The JADA® System is intended to provide control and treatment of abnormal postpartum uterine bleeding or hemorrhage when conservative management is warranted.
For general inquiries or to connect with a JADA Rep: jadainfo@organon.com
For existing customer support: USDirectCS@organon.com
Reference
1. D’Alton ME, Rood KM, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstet Gynecol. 2020;136(5):882-891. doi:10.1097/AOG.0000000000004138