About LIVIAL® (2.5mg tibolone)
LIVIAL® (2.5mg tibolone) is indicated for management of oestrogen-deficiency symptoms in postmenopausal women, more than one year after menopause1
After a woman’s last menstruation, a sharp decline in oestrogen plasma levels occurs, leading to a variety of symptoms,2 including:3
- vasomotor symptoms such as hot flushes
- disrupted sleep (with or without night sweats)
- mood changes such as depression
- vaginal dryness and dyspareunia
- muscle and joint ache
Besides these symptoms, oestrogen deficiency can result in osteoporosis — the most prevalent disease in postmenopausal women, often resulting in poor quality of life. 4
LIVIAL® offers benefits to postmenopausal women experiencing oestrogen-deficiency symptoms1
In postmenopausal women taking hormone replacement therapy (HRT), LIVIAL® vs. conjugated oestrogen/progestrogen group:
relieved climacteric* symptoms (at 6 months from baseline, n=77)5
improved vaginal atrophy (at 6 years from baseline, n=39)6
maintained bone mass density (over 2 years from baseline, n=35)7
*Climacteric symptoms were determined as5: hot flushes, sweating, parathesia, insomnia, vaginal dryness, dysphoria, palpitations of the heart, headache, nervousness, vertigo, mood depression, mood disorder, loss of libido, dryness of the skin, and muscle-joint-bone ache.
Read about the safety and tolerability profile of LIVIAL below:
The available data from randomised controlled trials are conflicting; however, observational studies have consistently shown that women who are prescribed LIVIAL in normal clinical practice are at an increased risk of having endometrial cancer diagnosed. In these studies risk increased with increasing duration of use. LIVIAL increases endometrial wall thickness, as measured by transvaginal ultrasound.
Breakthrough bleeding and spotting may occur during the first months of treatment. Women should be advised to report any breakthrough bleeding or spotting if it is still present after 6 months of treatment, if it starts beyond that time or if it continues after treatment has been discontinued. The woman should be referred for gynaecological investigation, which is likely to include endometrial biopsy to exclude endometrial malignancy.
Common
• Lower abdominal pain
• Abnormal hair growth
• Vaginal discharge
• Endometrial wall thickening
• Postmenopausal haemorrhage
• Breast tenderness
• Genital pruritus
• Vaginal candidiasis
• Vaginal haemorrhage
• Pelvic pain
• Cervical dysplasia
• Genital discharge
• Vulvovaginitis
• Weight increase
• Abnormal cervical smear*
Uncommon
• Oedema †
• Abdominal discomfort †
• Acne
• Breast discomfort
• Fungal infection
• Vaginal mycosis
• Nipple pain
*The majority consisted of benign changes. Cervix pathology (cervical carcinoma) was not increased with LIVIAL compared to placebo. 1
† These adverse reactions were identified through post-marketing surveillance. The frequency category was estimated based on relevant clinical trials.
A meta-analysis of epidemiological studies, including the Million Women Study (MWS), showed a significant increase in the risk of breast cancer in association with use of 2.5 mg LIVIAL.1
According to a meta-analysis based on 108,647 postmenopausal women who developed breast cancer (1992-2018):8
– all types of menopausal hormone therapy (MHT), other than vaginal oestrogens, were associated with an excess risk of breast cancer
– risk increased as duration of MHT use increased
– risk was greater for patients who received oestrogen-progestogen compared with oestrogen alone
– after discontinuing MHT, some excess risk persisted for over 10 years
How to initiate or change women from a continuous or
sequential HRT onto LIVIAL1
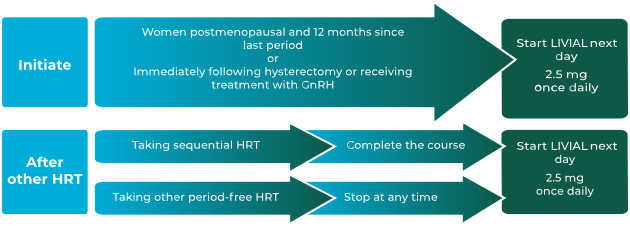
LIVIAL is indicated for:1
- the treatment of oestrogen-deficiency symptoms in postmenopausal women, more than 1 year after menopause
- the prevention of osteoporosis in postmenopausal women at high risk of future fractures who are intolerant of, or contraindicated for, other medicinal products approved for the prevention of osteoporosis
For all women, the decision to prescribe LIVIAL should be based on an assessment of the individual patient’s overall risks and, particularly in the over 60s, should include consideration of the risk of stroke.1
The images are for illustrative purposes only. The women shown are models who have granted permission for their image to be used
References
- LIVIAL Summary of Product Characteristics.
- Landgren M B, Helmond F A, Engelen S. Tibolone relieves climacteric symptoms in highly symptomatic women with at least seven hot flushes and sweats per day. Maturitas 2005;50(3):222-230.
- Santoro N, Epperson C N and Mathews S B. Menopausal symptoms and their management. Endocrionl Metab Clin North Am 2015;44(3):497-515.
- Ji M X, Yu Q. Primary osteoporosis in postmenopausal women. Chronic Dis Transl Med 2015;1(1):9-13.
- Egarter C, Huber J et al. Tibolone versus conjugated estrogens and sequential progestogen in the treatment of climacteric complaints. Maturitas 1996;23(1):55-62.
- Morris E P, Wilson P O et al. Long term effects of tibolone on the genital tract in postmenopausal women. Br J Obstet Gynaecol 1999;106(9):954-959.
- Lippuner K, Haenggi W et al. Prevention of postmenopausal bone loss using tibolone or conventional peroral or transdermal hormone replacement therapy with 17beta-estradiol and dydrogesterone. J Bone Miner Res 1997;12(5):806-812.
- Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019;394(10204):1159-1168.
Supporting documentation
Prescribing Information | Summary of Product Characteristics | Patient Information Leaflet
By clicking the above links you will leave the Organon website and be taken to external websites.

