
Click here for prescribing informationNEXPLANON® (etonogestrel 68 mg implant) is indicated for contraception.
Safety and efficacy have been established in women between 18 and 40 years of age.1

Click here for prescribing informationNEXPLANON® (etonogestrel 68 mg implant) is indicated for contraception.
Safety and efficacy have been established in women between 18 and 40 years of age.1
The implant for Nexplanon® is shown in this image (not to scale). Actual implant length is 4 cm¹.
From the 1st of January 2025, GP practices will be able to order, keep in stock and administer Nexplanon® to their patients as needed, and subsequently claim back the cost. This means that patients do not have to go to the pharmacy to pick up the implant and then make a further GP appointment to get it fitted.
To find out more, please book a meeting with your local Organon representative at askus@organon.com
Radiopaque1
Flexible1
Non-biodegradable1
Length: 4 cm, diameter: 2 mm1
Preloaded in a sterile, disposable applicator1
Containing 68 mg of etonogestrel1
The benefits of progestogen use should be weighed against the possible risk for each individual woman and should be discussed with the woman before she decides to start with NEXPLANON®. Please refer to the NEXPLANON® Summary of Product Characteristics for a full list of warnings and precautions for use¹.
Contraindications1:
Active venous thromboembolic disorder
Known or suspected sex steroid sensitive malignancies
Presence or history of liver tumours (benign or malignant)
Presence or history of severe hepatic disease as long as liver function values have not returned to normal
Undiagnosed vaginal bleeding
Hypersensitivity to the active substance or to any of the excipients
Wherever life takes her, plan on with NEXPLANON®
To learn more, please contact us at askus@organon.com
to book a meeting with your local Organon representative.
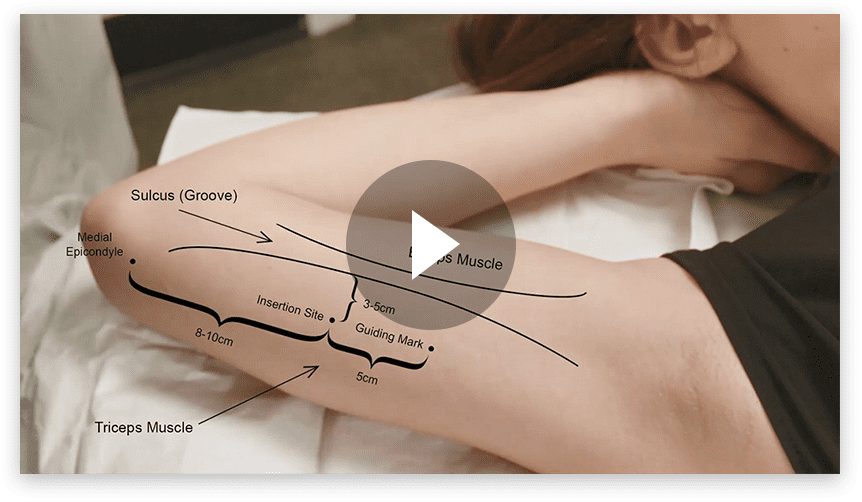
P = Proximal D = Distal
…at the inner side of the non-dominant upper arm, overlying the triceps muscle about 8-10 cm from the medial epicondyle of the humerus and 3-5 cm posterior to the sulcus (groove) between the biceps and triceps muscles.1
For additional information on NEXPLANON® insertion or removal, please refer to the videos below, or to the Summary of Product Characteristics.

Click here for Prescribing Information
Insertion and removal should be performed under aseptic conditions and only by HCPs who have completed training for the use of the NEXPLANON® applicator and the techniques for insertion and removal.¹
Your training options are provided by the Faculty of Sexual and Reproductive Healthcare of the Royal College of Obstetricians and Gynaecologists (FSRH).
It is strongly recommended that NEXPLANON® be inserted and removed only by healthcare professionals (HCPs) who have completed training for the use of the NEXPLANON® applicator and the techniques for insertion and removal of the NEXPLANON® implant, and, where appropriate, that supervision be requested prior to inserting or removing the implant.
To learn more about your training options, please contact us at askus@organon.com.
References
Prescribing Information | Summary of Product Characteristics | Patient Information Leaflet
By clicking the above links you will leave the Organon website and be taken to external websites.